Plasmacluster Technology Demonstrates Effectiveness in Reducing Novel Coronavirus
July 15, 2021
Plasmacluster Technology Demonstrates Effectiveness in Reducing Novel Coronavirus (Including Variant)*1 Contained in Adherent Saliva, a World First*2
Confirmed the efficacy against adherent viruses under conditions where viral infectious titer*3 tends to remain intact
In a world first, Sharp Corporation has demonstrated that the infectious titer of a novel coronavirus (SARS-CoV-2) including the variant strain contained in adherent saliva can be reduced more than 99.4% by exposing to Plasmacluster ions for two hours at 60% humidity*4, which is the recommended condition for countering viral infections. This achievement was accomplished under the supervision of Professor Hironori Yoshiyama of the Department of Microbiology, Shimane University Faculty of Medicine (a Board member of the Japanese Society for Virology), Professor Shigeru Watanabe, Meikai University School of Health Sciences, and Professor Masashi Yamakawa, Department of Mechanical and System Engineering, Kyoto Institute of Technology.
In general, the route of infection of SARS-CoV-2 is thought to be broadly divided into infection by droplet including aerosols of airborne virus particles, and infection through contact with the virus adhering to surfaces. Accordingly, in September 2020, Sharp demonstrated the effectiveness of Plasmacluster technology in reducing airborne SARS-CoV-2, and this time, Sharp has newly demonstrated its effectiveness on reducing SARS-CoV-2 adhering to surfaces.
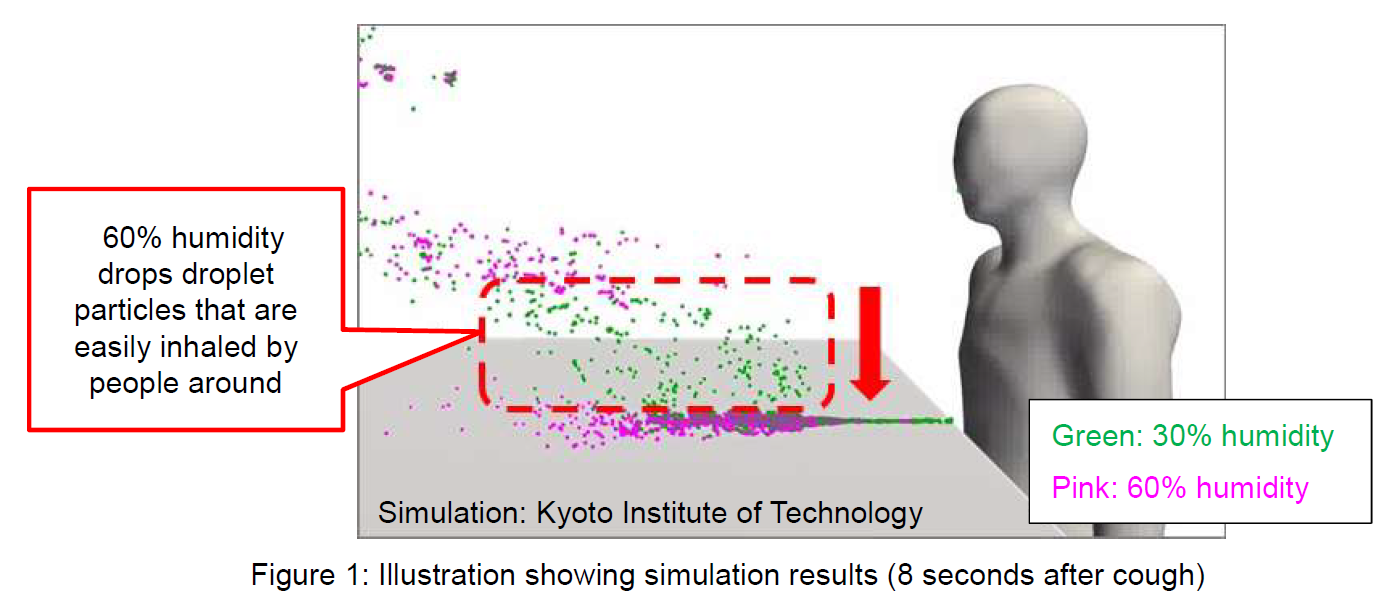
First, Sharp confirmed the movement of droplet particles at different humidity levels in a simulated real-life environment (Preliminary Verification [1] below). The results of simulating cases in which a person coughs in the environment with 30% humidity versus 60% showed that, fewer droplet particles were suspended in the air around the person in an environment with 60% humidity than 30%, but instead, the droplets fell down and adhered to the table. Based on this result, Sharp thought it's important to verify the effect of reducing the amount of SARS-CoV-2 that had fallen and adhered to a surface in an environment with 60% humidity where the risk of infection from airborne viruses is reduced by droplet particles dropping.
Next, since most of the droplets that cause the viral infection are derived from saliva, Sharp measured and compared the infectious titer in environment with 60% humidity between SARS-CoV-2 mixed with liquid medium which is commonly used for virus testing and SARS-CoV-2 mixed with saliva (Preliminary Verification [2]).
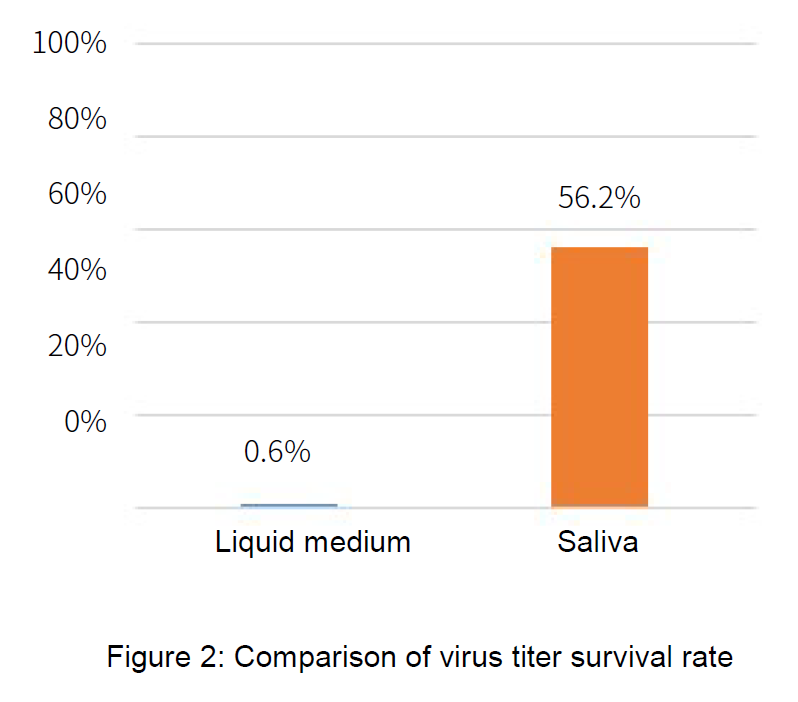
The result showed the infectious titer in the liquid medium left less than 1% after 2 hours, while the one in saliva, about 56% remained intact.
Based on these verification results, assuming that SARS-CoV-2 is present in adhering saliva at a humidity of 60% as in a real-life environment, the effect of Plasmacluster technology was verified. In conclusion, it was confirmed that the infectious titer including the variant strain was reduced more than 99.4%.
Sharp will continue to contribute to society by conducting a wide range of studies demonstrating the effectiveness of Plasmacluster technology.
Comments from Professor Hironori Yoshiyama, Department of Microbiology, Shimane University Faculty of Medicine.
In order to prevent virus infection, it is important to maintain the environment at a relative humidity of about 60% by humidification, thereby preventing the human respiratory tract mucosa from drying out, and maintaining the protective function, and to suppress the infectivity of the virus. However, while an environment with a relative humidity of 60% reduces the number of suspended droplet particles, airborne droplet particles fall down and adhere to surfaces. Therefore, it is also necessary to consider the countermeasures against adhering viruses. In this verification, Plasmacluster technology significantly inactivates SARS-CoV-2 contained in adherent saliva in an environment with 60% humidity where physiological protective function is maintained. The current result also shows its efficacy to the variant strain, which must be applied to new variants that will appear in the future.
- *1 Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): The strain of coronavirus that causes coronavirus disease 2019 (COVID-19). Variant strain is the alpha variant.
- *2 Ion-emission air purification technologies (as of July 15, 2021; based on Sharp research).
- *3 Number of infectious virus.
- *4 Relative humidity.
- ●Plasmacluster and the Plasmacluster logos are registered trademarks of Sharp Corporation.
- ■ Overview of Pre-Verification Tests
- ◆Preliminary Verification (1): Verification of airborne droplet particle dependence on humidity
- ●Verifying organization: Department of Mechanical and System Engineering, Kyoto Institute of Technology
- ●Verification method: Simulation of droplet particles generated by coughing while indoors
- ●Simulation conditions: Temperature of 20°C; relative humidity of 30% and 60%
- ●Results: It was confirmed that, at a relative humidity of 60%, while fewer droplet particles, which are easily inhaled, remain suspended in the air around the person than at a relative humidity of 30%, droplet particles will sink and adhere to the table.
- ◆Preliminary Verification (2): Verification of the effect of saliva on viral infectious titer
- ●Testing organization : Department of Microbiology, Shimane University Faculty of Medicine
- ●Verification method: Compare infectious titers after allowing SARS-CoV-2 mixed with liquid medium and with saliva, respectively, to stand undisturbed for two hours.
- ●Verification virus: Novel coronavirus SARS-CoV-2
- ●Test conditions: Temperature of approx. 20°C; relative humidity of approx. 60%
- (Liquid medium) D-MEM/Ham's F-12 nutrient mixture
- (Saliva) Saliva from seven person (men and women)
- (Test specimen) Apply 50 microliters of liquid medium or saliva containing virus on each filter.
- (Evaluation method) TCID50 assay*
- * The TCID50 assay is a method to check viral infectious titer by inoculating cells using a stepwise diluted virus solution.
- ●Results: It was confirmed that the infectious titer in the liquid medium was less than 1%, while the infectious titer in saliva remained at about 56%.
Table 1: Infectious titer (TCID50/ml) and survival rate after natural attenuation for two hours
| Initial value | After two hours | Survival rate | |
|---|---|---|---|
| Liquid medium | 1.0×107 | 5.6×104 | 0.6% |
| Saliva | 1.0x107 | 5.6x106 | 56.2% |
- ■ Overview of Test to Verify Effectiveness of Plasmacluster Technology
- ●Testing organization: Department of Microbiology, Shimane University Faculty of Medicine
- ●Verification apparatus: Adherent virus test device equipped with Plasmacluster technology
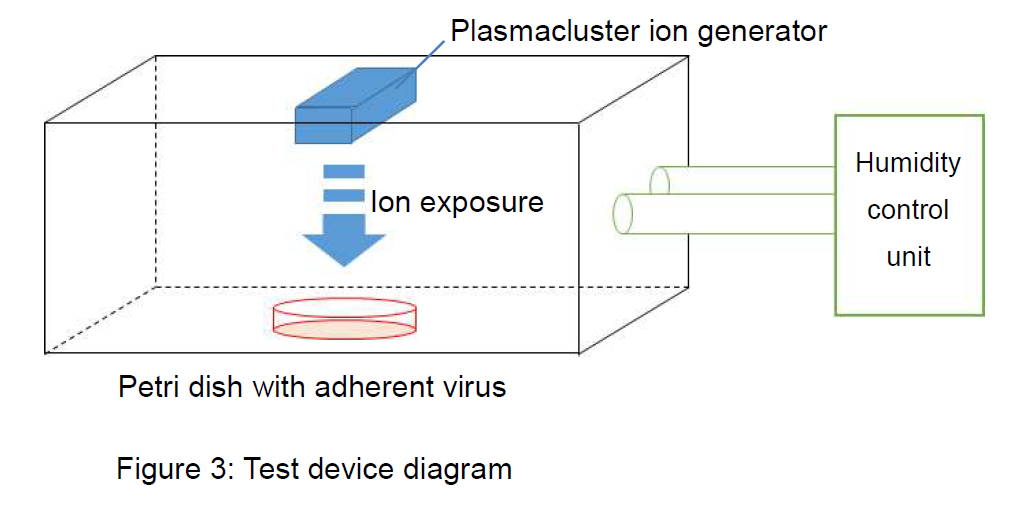
- ● Plasmacluster ion density: Approx. 600,000/cm3 (ion exposure distance: 10 cm)
- ●Test space: Approx. 38 liters
- ●Test conditions: Temperature of approx. 20°C; relative humidity of approx. 60%
- ●Control test: Comparison using the device described above without Plasmacluster ion generation
- ●Verification virus: Novel coronavirus SARS-CoV-2 and variant strain (Alpha variant)
- ●Test procedure:
- (1) Mix saliva into virus solution
- (2) Apply 50 microliters virus solution on the filter, expose to Plasmacluster ions for two hours, and then recover.
- (3) Calculate viral infectious titer (TCID50/ml) from the recovered virus solution using TCID50 assay.
- ●Results:
Table 2: Infection titer (TCID50/ml) and reduction rate of new coronavirus contained in adherent saliva
| Verification virus | Without Plasmacluster ions | With Plasmacluster ions | Reduction | |
|---|---|---|---|---|
| (a) | SARS-CoV-2 | 5.6x106 | 1.8x104 | 99.7% |
| (b) | SARS-CoV-2(Alpha variant) | 5.6x104 | 3.2×102 | 99.4% |

■ Research Institutes That Provided Data for Sharp's Academic Marketing
| Target | Testing and Verification Organization |
|---|---|
| Efficacy proven in clinical trials | Graduate School of Medicine, University of Tokyo / Public Health Research Foundation |
| Faculty of Science and Engineering, Chuo University / Clinical Research Support Center, University Hospital, University of Tokyo | |
| Animal Clinical Research Foundation | |
| Soiken Inc. | |
| School of Bioscience and Biotechnology, Tokyo University of Technology | |
| National Trust Co., Ltd. / HARG Treatment Center | |
| National Center of Tuberculosis and Lung Diseases, Georgia | |
| Dentsu ScienceJam Inc. | |
| Littlesoftware Inc. | |
| National Institute of Fitness and Sports in Kanoya | |
| Viruses | Kitasato Research Center of Environmental Sciences |
| Seoul National University | |
| Shanghai Municipal Center for Disease Control and Prevention, China | |
| Kitasato Institute Medical Center Hospital | |
| Retroscreen Virology, Ltd., UK | |
| Shokukanken Inc. | |
| University of Indonesia | |
| Hanoi College of Technology, Vietnam National University, Vietnam | |
| Institut Pasteur, Ho Chi Minh City, Vietnam | |
| National Research Center for the Control and Prevention of Infectious Diseases, Institute of Tropical Medicine, Nagasaki University | |
| Department of Microbiology, Shimane University, Faculty of Medicine | |
| Allergens | Graduate School of Advanced Sciences of Matter, Hiroshima University |
| Department of Biochemistry and Molecular Pathology, Graduate School of Medicine, Osaka City University | |
| Fungi | Ishikawa Health Service Association |
| University of Lübeck, Germany | |
| Professor Gerhard Artmann, Aachen University of Applied Sciences, Germany | |
| Japan Food Research Laboratories | |
| Shokukanken Inc. | |
| Shanghai Municipal Center for Disease Control and Prevention, China | |
| Biostir Inc. | |
| Medical Mycology Research Center, Chiba University | |
| Bacteria | Ishikawa Health Service Association |
| Shanghai Municipal Center for Disease Control and Prevention, China | |
| Kitasato Research Center of Environmental Sciences | |
| Kitasato Institute Medical Center Hospital | |
| Dr. Melvin W. First, Professor Emeritus, Harvard School of Public Health, US | |
| Animal Clinical Research Foundation | |
| University of Lübeck, Germany | |
| Professor Gerhard Artmann, Aachen University of Applied Sciences, Germany | |
| Japan Food Research Laboratories | |
| Shokukanken Inc. | |
| Chest Disease Institute, Thailand | |
| Biostir Inc. | |
| Odors, pet smells | Boken Quality Evaluation Institute |
| Animal Clinical Research Foundation | |
| Skin beautifying effects | School of Bioscience and Biotechnology, Tokyo University of Technology |
| Hair beautifying effects | Saticine Medical Co., Ltd. |
| C.T.C Japan Ltd. | |
| Plant | Facility of Agriculture, Shizuoka University |
| Hazardous chemical substances | Sumika Chemical Analysis Service Ltd. |
| Indian Institutes of Technology Delhi | |
| Working mechanism of inhibitory effects on viruses, fungi, and bacteria | Professor Gerhard Artmann, Aachen University of Applied Sciences, Germany |
| Working mechanism of inhibitory effects on allergens | Graduate School of Advanced Sciences of Matter, Hiroshima University |
| Working mechanism of skin moisturizing (water molecule coating) effect | Research Institute of Electrical Communication, Tohoku University |







